Protect Brands, Maintain Trust, and Prevent Losses
Building trust in a brand is a challenge. Maintaining it is even harder. Atlantic Zeiser’s solutions allow for brand protection and for monitoring distribution channels. Also, by turning anonymous mass-produced goods into unique products, they enable your customers to engage directly with your brands.
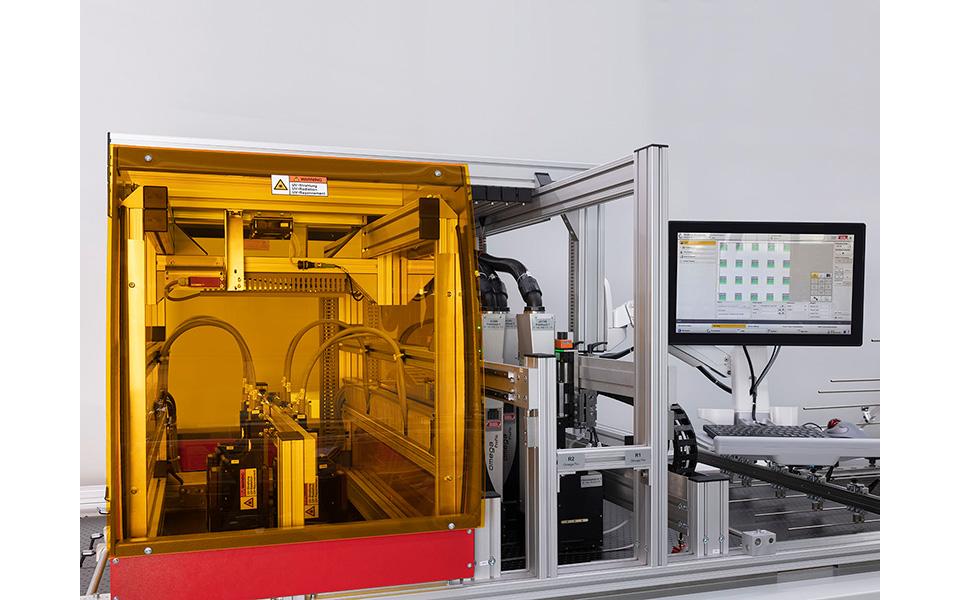
Over a pool of existing technological components, bespoke modules dedicated to your needs to identify uniquely your products. These modules make your products forgery-proof. Quality controls implemented along your processes, from manufacturing to the end consumer, leave product and brand piracy behind.